Why is Acid Stratification a Killer of lead-acid batteries?
High Deep Cycle demands on battery-powered equipment, the increased cyclic demand and parasitic electrical loads brought about by the use of start-stop technologies, increased electrical systems in modern vehicles, and the frequent partial state of charge (PSOC) operation combine to accelerate the #1 cause of declining battery life expectancy: Acid Stratification.
Acid stratification happens naturally in flooded lead-acid batteries. The fluid in a battery is called the electrolyte. The electrolyte is a mixture of sulfuric acid and water. Acid is heavier than water and is fundamental to the electrochemical charge and discharge process in a lead-acid battery. Acid stratification happens when the heavier acid in the battery’s electrolyte separates from the water and assembles at the bottom of the battery’s cell, creating an area of very high specific gravity electrolyte.
What are the Effects of Acid Stratification?
- In wet flooded batteries, acid stratifies (sinks to the bottom of the battery’s cells). The upper portion of the battery’s plates is left subject to low specific gravity electrolyte (now mostly water). The upper portion of the plate is rendered inactive and is no longer capable of supporting discharge capacity. Under these conditions, the useful active material in the battery will be reduced by as much as 40% within six months of normal use, creating “dead lead” or “inactive active material.” (See Figure 1)
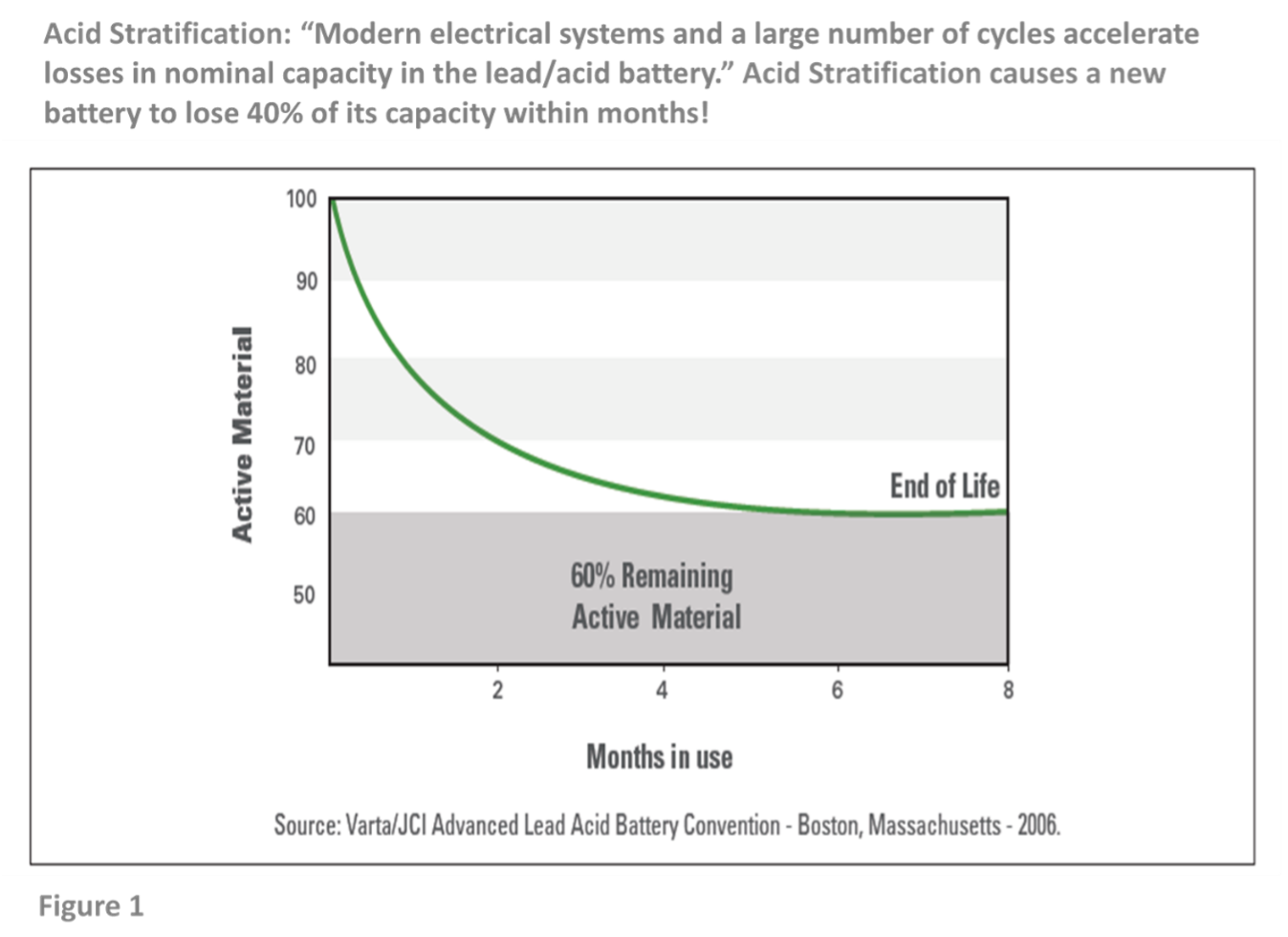
- The stratified acid at the bottom of the battery’s cell focuses discharge activity to the bottom of the cell, causing the bottom part of the plate to work overtime. While the bottom part of the plate gets excessively discharged, the top part of the plate receives most of the charging activity. As a result, acid stratification can cause a battery’s dynamic charge acceptance1 (“DCA”) to decline by 50% to 70% within six months of installation, increasing alternator wear and tear and decreasing fuel efficiency.
- Since electrical current moves more easily through water (top part of the cell) than it does through acid (bottom part of the cell), stratified acid concentrates charging current and charging heat at the upper part of the plate, accelerating corrosion which dramatically lowers the battery’s cranking power (“CCA”).
- Stratified acid promotes increased internal resistance, lower conductivity and accelerated sulfation on the lower part of the plates, reducing the battery’s dynamic charge acceptance. This means a sulphated battery will only accept a surface charge, resulting in a false positive state of charge readings to vehicle computers and battery testers. So, a battery may appear fully charged but only provide low “CCA” and “AH”/”RC.”
Acid stratification is accelerated if:
- the battery operates in a partial state of charge (PSOC)
- the battery seldom receives a full charge
- the battery is constantly micro-cycled between 3% - 17.5% DOD as in start-stop vehicles
- the battery is regularly high cycled between 17.5% and 30%
- the battery is regularly deep-cycled beyond 50% DOD
- the battery is used or exposed to extreme temperatures,
- and the battery is left standing for long periods.
All of these conditions contribute to premature battery failure.
